Phytotherapeutic Support of Thyroid Function
© Dr Joseph J Collins, RN, ND, 2007 – 2016 First published in NutraNews, Jan, 2007. This on-line version contains some updated material.
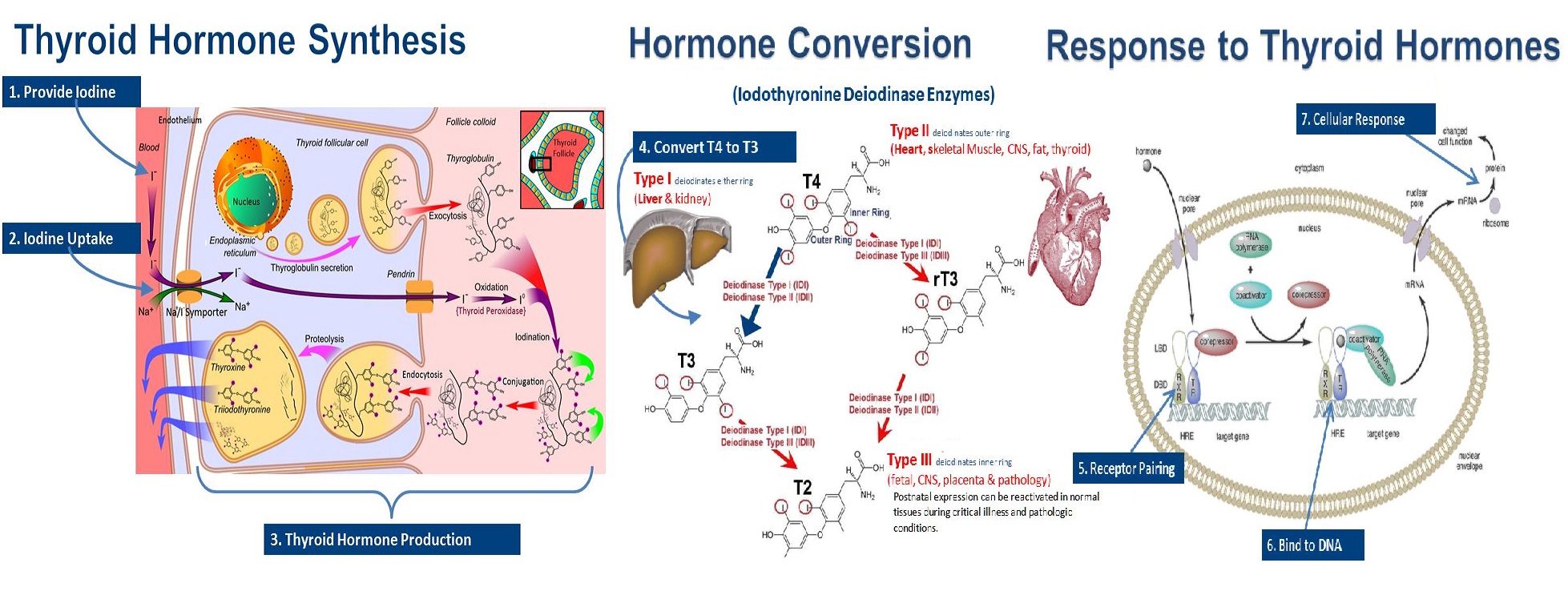
Proper production and function of thyroid hormones involves a number of complex interdependent processes that must all occur to optimal thyroid health. There are seven specific requirements for the production and function of thyroid hormones. Each of these actions is required to maintain optimal thyroid hormone health. These processes are all occurring simultaneously in different parts of the thyroid, as well as tissues and cells throughout the body.
Phytotherapy is the scientific study on the effects and clinical use of herbal medicines. The choice of phytotherapeutic agents can be made based on years of clinical experience, and validation by review of international scientific/medical literature. Specific phytotherapeutic agents, when properly combined, can significantly improve the function of all seven critical actions required for thyroid hormone health. Critical to effective use of herbs is the understanding that they are used to support and restore normal function to processes that occur naturally.
Provide Safe Bio-Available Iodine for Thyroid Cells (Step 1)
Step one involves the safe consistent intake of dietary bio-available iodine in the proper amount. The question of what is the “right amount” is best answered by looking at objective, scientific data. Based on studies reviewed by a panel or researchers at the Institute of Medicine, the Estimated Average Requirement (EAR) for iodine is 95 mcg/day for men and women 19 y/o and above. [1] The studies that were used actually measured how much iodine the body would retain for use each day.
The three studies revealed similar data on average iodine uptake (96.5 mcg/day, 91.2 mcg/day & 97 mcg/day), which was averaged to the 95 mcg/day. The first thing that should be noted is that all three studies gave very similar results. Therefore, we can be pretty confident that adult humans use somewhere between 91 and 97 mcg of iodine per day.
Noting that this is the “average”, they also calculated the coefficient of variation (CV) at 40%, and increased the EAR by that amount (38 mcg/day), arriving at 133 mcg/day, which was rounded up to the nearest 50 mcg to provide an RDA of 150 mcg/day, an amount that covers 97 to 98 percent of people.
Now, while 150 mcg/day is a good guideline, the RDA is only half of the picture. It only tells us what the body uses, not how much it can tolerate. The Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals. For this, the researchers looked at a number of other studies,
They found a No-Observed-Adverse-Effect Level (NOAEL) of 1,00 to 1,200 mcg/day and a Lowest-Observed-Adverse-Effect Level (LOAEL) of 1,700 mcg/day. The uncertainty factor of 1.5 was derived from those numbers (actually 1.55). The LOAEL was divided by 1.5 to arrive at 1,133 mcg/day, which was rounded down to 1,100 mcg/day, which is the same as 1.1 mg/day.
We can now say that the average adults needs about 150 mcg of iodine a day, but not more than 1,100 mcg. I like to add another “CV” of 38 mcg to the 150 mcg of the RDA to cover a higher percentage of people. Rounding that 188 up to 200 mcg of iodine per day is still within the safety zone defined by the UL. So 200 mcg or iodine per day is a good, safe intake of bioavailable iodine.
Most supplement manufactures that follow the evidence based international scientific literature keep the iodine dosage within the safe range. Some proponents of extremely high dose iodine supplements may use opinion papers from non-scientific commercial newsletters to justify the high dosages that are sold to patients. The research is ongoing, but at this point in time there is some indication that more than adequate or excessive iodine intake may lead to hypothyroidism and autoimmune thyroiditis [2], and may be a risk factor for the development of papillary thyroid cancer. [3] Noting the possible risks, it is best to stay below the 1,100 mcg of iodine per day, which as noted is 1.1 mg/day.
Plants that Provide Safe Bio-Available Iodine for Thyroid Cells (Step 1)
There are phytotherapeutic agents that address the first step of proper thyroid function, and provide safe, bio-available iodine for the thyroid gland. Sea Kelp (Ascophyllum nodosum) and Bladderwrack (Fucus vesiculosus), are both excellent source of safe bio-available iodine. An additional benefit of Ascophyllum nodosum is its ability to increase glutathione peroxidase activity, an important antioxidant. Human thyrocytes synthesize and secrete extracellular glutathione peroxidase, which translocates into the intracellular space and prevents peroxidative damage of thyrocytes from diffusion of extracellular H202 (hydrogen peroxide) during stimulation of thyroid-hormone synthesis [4]. This hydrogen peroxide can cause the inflammation that interferes with uptake of iodine, and can actually promote thyroid autoimmune disease. Ascophyllum nodosum may therefore decrease occurrence of autoimmune thyroid disease, since thyrocytes exposed to locally increased H202 increase the risk autoimmune thyroid disease [5].
Bladderwrack (Fucus vesiculosus), another dietary source of natural iodine also demonstrates anti-estrogen properties in both human and animal studies, suggesting that it may contribute protective health to estrogen sensitive tissues [6] [7]. This is important because excessive local estrogens can cause inflammation of the thyroid. The estrogen protecting properties of Fucus vesiculosus is of benefit to both men and women.
Ascophyllum nodosum and Fucus vesiculosus both provide fucoidan a sulfated polysaccharide that has a wide variety of biological activities including antioxidant, anti-thrombotic, anti-inflammatory and anti-autoimmune effects [8] [9] [10].
Collectively, Ascophyllum nodosum and Fucus vesiculosus, are able to provide safe, bio-available iodine while also protecting the thyroid cells, and decreasing the risk of developing autoimmune thyroid disease. Three ThyroMend™ capsules provide 200 mcg of iodine, the clinically effective dosage.
Iodine Uptake by Thyroid Cells (Step 2)
Step two is iodine being taken up by thyroid cells. This uptake of the iodine by thyroid cells (thyrocytes) specifically requires proper function of proteins on thyroid cells called sodium-iodide-symporter (NIS) proteins. Thyroid follicular cells transport iodide from blood into the follicular lumen against an iodide gradient (against the flow) by means of coupled transport of Na+ ions and I- ions via NIS proteins under the influence of TSH. The proinflammatory cytokines, IL-1alpha, IL-1beta, IL-6, and TNF-alpha have each demonstrated the ability to decrease TSH induced expression of NIS proteins [11] [12] Herein lies the next impedance to optimal thyroid function – inflammation reduces uptake of iodide by thyrocytes.
While phytotherapeutic agents which provide dietary iodine support the first step in hormonogenesis, the support of NIS protein function and control of proinflammatory cytokines are also required to promote optimal thyroid function in these initial steps of thyroid hormonogenesis.
Plants that Promote Iodine Uptake by Thyroid Cells (Step 2)
Phytotherapeutics that address the second step of proper thyroid function increase the uptake of iodine by thyroid cells. Taking iodine supplements is not enough; the iodine must get into the thyroid cells. This means concentrating iodine within thyroid cells, which is dependent on specific proteins working properly. Humulus lupulus (Hops), contains xanthohumol, a chalcone that enhances uptake of iodine into the thyroid gland by activation of the sodium-iodide-symporter (NIS) proteins [13]. Xanthohumol also repressed activation of NF-kappaB, thereby decreasing the expression of proinflammatory cytokines such as TNF-alpha and IL-6, which as noted, can interfere with function of NIS proteins [14] [15].
Coleus (Coleus forskohlii) contains forskolin, which is specifically able to mimic the effect of TSH in regard to iodide uptake, organification of iodine, thyroglobulin (TG) production, and promote secretion of T3 & T4, through an increase in the expression of sodium/iodide symporter (NIS) proteins [16] [17] [18].
ThyroMend™ contains clinically effective dosages of Humulus lupulus and Coleus forskohlii to enhance proper uptake of iodine into thyroid cells (thyrocytes).
T3 & T4 Production and Secretion from Thyrocytes (Step 3)
Step three includes both the production of thyroid hormones by thyrocytes, and the secretion of thyroid hormone from those cells. The production of thyroid hormones by thyrocytes typically begins with the sulfation of tyrosine residues in thyroglobulin, a process which is under the control of TSH [19] [20]. There is a close correlation between the sulfated tyrosine content of thyroglobulin and the production of thyroid hormones.
The sulfated tyrosine is then acted upon by thyroperoxidase (TPO), an enzyme mainly expressed in the thyroid that binds iodine onto the tyrosine residues on thyroglobulin for the production of thyroxine (T4) or triiodothyronine (T3), a process called “organification of iodine” [21].
Plants that Promote T3 & T4 Production and Secretion from Thyroid Cells (Step 3)
A few phytotherapeutic extract work together to promote the actual manufacturing and secretion of thyroid hormones. Thyroid hormone production requires attaching iodine atoms to organic proteins – a process rightly called organification of iodine. As noted, Coleus (Coleus forskohlii) mimics the effect of TSH in regard to iodide uptake, organification of iodine, thyroglobulin (TG), T4 & T3 production, and also promotes secretion of T3 & T4. Consequentially, Coleus extracts play an important role in these two steps of thyroid function [22].
Bacopa monniera (also known as Brahmi) exhibits thyroid stimulating abilities through an increase of T4 serum concentrations in animal studies. The increase of T4 by 41% without any notable increase in T3 or hepatic activity suggests that the action of Brahmi has more to do with direct thyroid stimulating activity than it does with hepatic conversion to T3 [23].
Ashwagandha (Withania somnifera) is another plant that directly affects production of thyroid hormones. It has the ability to directly act on thyroid tissue to bring about a rise in serum levels of thyroid hormones [24] [25]. In fact, there was even a case where a woman who took extremely high dosages of ashwagandha actually had an excessive rise of her thyroid hormone levels and had to lower dosage [26]. So, Withania somnifera can absolutely increase serum levels of thyroid hormone in humans, though excessive dosages should be avoided.
ThyroMend™ contains clinically effective dosages of Coleus forskohlii, Bacopa monniera and Withania somnifera which work together to support the optimal function of thyroid hormone production and secretion by thyrocytes.
Conversion of T4 to T3, with Decreased Revere T3 (rT3) Production (Step 4)
Step four involves promoting conversion of T4 to the more potent T3, and decreasing the amount of T4 that gets converted to reverse T3 (rT3). The thyroid hormone thyroxine (T4) is converted to the more active form triiodothyronine (T3) by the 5’- iodothyronine deiodinase (5’DI) enzyme. Inhibition of 5’DI is associated with decreased production of T3, and a relative increase of reverse T3 (rT3), a relatively inactive form of the hormone.
This relative elevation of rT3 levels with suppression of T3 is associated with clinical presentation of hypothyroidism, despite normal to elevated thyroxine (T4), and normal TSH levels [27]. This shift in thyroid hormone metabolism, with increased rT3/T3 ratio, has been associated with inactivation of type I 5’-iodothyronine deiodinase (5’DI) enzyme, by NFkappaB [28] [29] [30]. Activation of NF-kappaB also leads to increased expression of proinflammatory cytokines such as TNF-alpha and IL-6 which moderately decrease 5’DI activity [31] [32]
Plants that Increase Conversion of T4 to T3, and Decrease reverse T3 Production (Step 4)
Phytotherapeutic agents targeted to support optimal thyroid hormone metabolism towards T3 and away from reverse T3 (rT3), include agents which directly increase iodothyronine deiodinase activity, such as forskolin from Coleus forskohlii [33] [34], and agents which preserve iodothyronine deiodinase activity by decreasing NF-kappaB activation, such as xanthohumol from Humulus lupulus, guggulsterones from Commiphora mukul, Carnosol from Rosmarinus officinalis, and withanolides from Withania somnifera [35] [36] [37] [38] [39]. In addition to decreasing NF-kappaB activation, guggulsterones also directly stimulate triiodothyronine (T3) production through its action on liver enzymes, while also increasing the activity of endogenous antioxidants [40].
ThyroMend™ contains clinically effective dosages of Coleus forskohlii, Humulus lupulus, Commiphora mukul, Rosmarinus officinalis and Withania somnifera, which all support the important step of converting T4 to the more active T3, while opposing the production of the less potent reverse T3 (rT3).
The Last Three Steps: Cell Signaling
The final three steps in proper thyroid function involve a field of science called cell signaling biology. Simply put, cell signaling is exactly what it sounds like - how the cells throughout the body receive the signal from the thyroid. Basically, it involves hormones attaching to receptors which in turn go to the DNA and tell the cell what to do. When cells do not respond appropriately you can have hormone resistance. The most widely known example of hormone resistance is insulin resistance, which can lead to diabetes. Thyroid resistance can also be a problem. {More on cell signaling.}
Promote Thyroid Receptor Function (Step 5)
Step five involves maintaining enough functioning thyroid hormone receptors. In technical terms, thyroid hormone receptors (TR) are nuclear receptors involved in the regulation of cellular response to the thyroid hormone triiodothyronine (T3). So they are receptors that are on the nucleus of cells, that prefer to respond to T3, the most active of the thyroid hormones. Cellular response takes place after TRs allow T3 binding to T3 response elements (TRE) in target genes within the nuclear DNA. The TRs help “carry” the signal to specific a place (element) on the DNA within the nucleus. However, before TRs can work, they must attach themselves to another receptor called RXR. In fact, the TR receptors are totally dependent on RXR receptors to work. [41] [42] [43]. So TR function is dependent on having adequate RXR receptors. Without RXR, the TR will not function.
Plants that Promote Thyroid Receptor Function (Step 5)
Phytotherapeutic agents that support optimal thyroid hormone function by promoting the availability and function of RXR receptors include Rosemary (Rosmarinus officinalis) and Sage (Salvia officinalis) which provide carnosic acid, a polyphenolic diterpene that at low concentrations increases the number of RXR receptors [44] [45]. As noted, before TRs can work, they must attach themselves to another receptor called RXR. With adequate RXR function, the TR is able to go through the next step of heterodimerization.
ThyroMend™ contains clinically effective dosages of Rosemary (Rosmarinus officinalis) and Sage (Salvia officinalis) which work together to promote optimal thyroid receptor function.
Support Receptor Coupling (Step 6)
Step six involves the thyroid hormone receptor (TR) coupling or pairing to an Retinoid-X-receptor (RXR) in a process called heterodimerization. Heterodimerization is the process of two (di) different (hetero) proteins merging together to create a heterodimer, such as the RXR/TR heterodimer. It is the RXR/TR heterodimer that allows the signal from the thyroid hormone to be delivered to the DNA. As you might expect, the RXR receptor also forms dimmers with the retinoic acid receptor (RAR) (vitamin A). it also forms dimmers with the vitamin D receptor (VRD), as well as other hormone receptors. This coupling of receptors is required to allow thyroid hormones (and other hormones) to enter target cells and affect the hormone/receptor complex on target genes of the DNA. The RXR/TR heterodimer is required for T3 to target genes within target cells throughout the body [41] [42] [43]. The RXR/TR heterodimerization “gives permission” for the thyroid hormone to approach the DNA.
Plants that Support Receptor Coupling (Step 6)
Phytotherapeutic agents which support the coupling the two receptor elements (TR & RXR) accomplish this by decreasing inflammatory processes that interfere with receptor function, and specifically promote thyroid function. As previous noted certain phytotherapeutic agents can decrease NF-kappaB activation including xanthohumol from Humulus lupulus, guggulsterones from Commiphora mukul, carnosol from Rosmarinus officinalis, and withanolides from Withania somnifera [46] [47] [48] [49] [50]. This ability to specifically protect thyroid receptor function from being shut down because of inflammatory processes increases RXR/TR heterodimerization and allows the RXR/TR heterodimer to target gene expression.
ThyroMend™ThyroMend™ contains clinically effective dosages of Humulus lupulus, Commiphora mukul, Rosmarinus officinalis, and Withania somnifera to support thyroid receptor coupling and control inflammation that could interfere with the coupling process.
Promote Expression in Target Genes (Step 7)
Step seven involves activators and coactivators within the nucleus that – as the name implies – activate the gene that is being targeted allowing the gene to express the action it is programmed to express. More specifically it works with the RXR/TR heterodimer to “co-activate” the gene. The gene expression may be a command to make proteins that affect metabolism, or proteins that command the production of neurotransmitters or other molecules, depending on the functions of the specific cell that is being activated by the thyroid hormone. This final step is appropriately called “gene expression”.
Plants that Support Target Gene Expression (Step 7)
Phytotherapeutic agents that are involved in the final process of instructing the gene to “express” itself should rightfully include all the plants that first helped promote heterodimerization because without the xanthohumol from Humulus lupulus, guggulsterones from Commiphora mukul, carnosol from Rosmarinus officinalis, and withanolides from Withania somnifera there would be no RXR/TR heterodimers.
Decreased NF-kappaB activation is important for receptor function So controlling NFkappaB in thyroid tissue is important to allow binding to the targeted DNA gene where RXR is a dimerization partner, such as the RXR/TR heterodimer [51]. The RXR/TR initiated gene expression may be further enhanced 2.5 to 3-fold by forskolin, which occurs in Coleus forskohlii [52] [53].
ThyroMend™ contains clinically effective dosages of Humulus lupulus, Commiphora mukul, Rosmarinus officinalis, and Withania somnifera which support optimal gene expression to that the cells affected by thyroid hormones can properly receive the hormone signal.
Conclusion
After gene expression it is the responsibility of the cell to do what it was instructed to do by the thyroid hormone. Of course if the cell is toxic or malnourished then it will not do as good a job as it should, so it is important to maintain the health of the cell. This can be accomplished by following the Hormone Health Guidelines with specific attention being given to the most fundamental health needs such as a healthy diet and lifestyle, and regular intake of a high-grade multiple vitamin-mineral formulation, as well as essential fatty acids.
Complete thyroid health requires both support of healthy thyroid tissue, and support of health in every tissue that is affected by thyroid hormones. This is best accomplished through the use of ThyroMend™, as discussed in the Hypothyroid Protocol.
Next: Learn the Hypothyroid Protocol.
References [PMID can be accessed at PubMed]
[1] Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press. 2001 [NAP Website]
[2] Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006 Jun 29;354(26):2783-93. [PMID: 16807415]
[3] Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009 May;94(5):1612-7. [PMID: 19190105]
[4] Saker KE, Fike JH, Veit H, Ward DL. Brown seaweed- (Tasco) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J Anim Physiol Anim Nutr (Berl). 2004 Apr;88(3-4):122-30. [PMID: 15059236]
[5] Duthoit C, Estienne V, Giraud A, Durand-Gorde JM, Rasmussen AK, Feldt-Rasmussen U, Carayon P, Ruf J. Hydrogen peroxide-induced production of a 40 kDa immunoreactive thyroglobulin fragment in human thyroid cells: the onset of thyroid autoimmunity? Biochem J. 2001 Dec 15;360(Pt 3):557-62. [PMID: 11736644]
[6] Skibola CF, Curry JD, VandeVoort C, Conley A, Smith MT. Brown kelp modulates endocrine hormones in female sprague-dawley rats and in human luteinized granulosa cells. J Nutr. 2005 Feb;135(2):296-300. [PMID: 15671230]
[7] Skibola CF. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: a case report. BMC Complement Altern Med. 2004 Aug 4;4:10. [PMID: 15294021]
[8] Chevolot L, Mulloy B, Ratiskol J, Foucault A, Colliec-Jouault S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr Res. 2001 Feb 28;330(4):529-35. [PMID: 11269406]
[9] Yang JW, Yoon SY, Oh SJ, Kim SK, Kang KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2006 Jul 21;346(1):345-50. [PMID: 16756944]
[10] Gal I, Bajnok E, Szanto S, Sarraj B, Glant TT, Mikecz K. Visualization and in situ analysis of leukocyte trafficking into the ankle joint in a systemic murine model of rheumatoid arthritis. Arthritis Rheum. 2005 Oct;52(10):3269-78. [PMID: 16206283]
[11] Schumm-Draeger PM. Sodium/iodide symporter (NIS) and cytokines. Exp Clin Endocrinol Diabetes. 2001;109(1):32-4. [PMID: 11573136]
[12] Ajjan RA, Watson PF, Findlay C, Metcalfe RA, Crisp M, Ludgate M, Weetman AP. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J Endocrinol. 1998 Sep;158(3):351-8. [PMID: 9846164]
[13] Radovic B, Schmutzler C, Kohrle J. Xanthohumol stimulates iodide uptake in rat thyroid-derived FRTL-5 cells. Mol Nutr Food Res. 2005 Sep;49(9):832-6. [PMID: 16092068]
[14] Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J. 2006 Mar;20(3):527-9. [PMID: 16403733]
[15] Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2006 Mar 22. [PMID: 16563612]
[16] Hiraiwa M. Adenylate cyclase system responsive to thyroid stimulating hormone (TSH) of porcine thyroid cells in primary monolayer cultures. Potential effect of forskolin on TSH-mediated adenylate cyclase stimulation. Nippon Naibunpi Gakkai Zasshi. 1987 Jan 20;63(1):34-44. [PMID: 3030831]
[17] van Sande J, Cochaux P, Dumont JE. Forskolin stimulates adenylate cyclase and iodine metabolism in thyroid. FEBS Lett. 1982 Dec 13;150(1):137-41. [PMID: 6297967]
[18] Venkateswaran A, Marsee DK, Green SH, Jhiang SM. Forskolin, 8-Br-3',5'-cyclic adenosine 5'-monophosphate, and catalytic protein kinase A expression in the nucleus increase radioiodide uptake and sodium/iodide symporter protein levels in RET/PTC1-expressing cells. J Clin Endocrinol Metab. 2004 Dec;89(12):6168-72. [PMID: 15579773]
[19] Nlend MC, Cauvi D, Venot N, Chabaud O. Sulfated tyrosines of thyroglobulin are involved in thyroid hormone synthesis. Biochem Biophys Res Commun. 1999 Aug 19;262(1):193-7. [PMID: 10448091]
[20] Cauvi D, Venot N, Nlend MC, Chabaud OM. Thyrotropin and iodide regulate sulfate concentration in thyroid cells. Relationship to thyroglobulin sulfation. Can J Physiol Pharmacol. 2003 Dec;81(12):1131-8. [PMID: 14719032]
[21] Nlend MC, Cauvi DM, Venot N, Chabaud O. Role of sulfated tyrosines of thyroglobulin in thyroid hormonosynthesis. Endocrinology. 2005 Nov;146(11):4834-43. [PMID: 16037381]
[22] Laurberg P. Forskolin stimulation of thyroid secretion of T4 and T3. FEBS Lett. 1984 May 21;170(2):273-6. [PMID: 6327383]
[23] Kar A, Panda S, Bharti S. Relative efficacy of three medicinal plant extracts in the alteration of thyroid hormone concentrations in male mice. J Ethnopharmacol. 2002 Jul;81(2):281-5. [PMID: 12065164]
[24] Panda S, Kar A. Changes in thyroid hormone concentrations after administration of ashwagandha root extract to adult male mice. J Pharm Pharmacol. 1998 Sep;50(9):1065-8. [PMID: 9811169]
[25] Panda S, Kar A. Withania somnifera and Bauhinia purpurea in the regulation of circulating thyroid hormone concentrations in female mice. J Ethnopharmacol. 1999 Nov 1;67(2):233-9. [PMID: 10619390]
[26] van der Hooft CS, Hoekstra A, Winter A, de Smet PA, Stricker BH. Thyrotoxicosis following the use of ashwagandha. Ned Tijdschr Geneeskd. 2005 Nov 19;149(47):2637-8. [PMID: 16355578]
[27] Jakobs TC, Mentrup B, Schmutzler C, Dreher I, Kohrle J. Proinflammatory cytokines inhibit the expression and function of human type I 5'-deiodinase in HepG2 hepatocarcinoma cells. Eur J Endocrinol. 2002 Apr;146(4):559-66. [PMID: 11916626]
[28] Mastorakos G, Pavlatou M. Exercise as a stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Horm Metab Res. 2005 Sep;37(9):577-84. [PMID: 16175498]
[29] Davies PH, Sheppard MC, Franklyn JA. Regulation of type I 5'-deiodinase by thyroid hormone and dexamethasone in rat liver and kidney cells. Thyroid. 1996 Jun;6(3):221-8. [PMID: 8837330]
[30] Kwakkel J, Wiersinga WM, Boelen A. Differential involvement of nuclear factor-kappaB and activator protein-1 pathways in the interleukin-1beta-mediated decrease of deiodinase type 1 and thyroid hormone receptor beta1 mRNA. J Endocrinol. 2006 Apr;189(1):37-44. [PMID: 16614379]
[31] Mastorakos G, Pavlatou M. Exercise as a stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Horm Metab Res. 2005 Sep;37(9):577-84. [PMID: 16175498]
[32] Jakobs TC, Mentrup B, Schmutzler C, Dreher I, Kohrle J. Proinflammatory cytokines inhibit the expression and function of human type I 5'-deiodinase in HepG2 hepatocarcinoma cells. Eur J Endocrinol. 2002 Apr;146(4):559-66. [PMID: 11916626]
[33] Tang KT, Braverman LE, DeVito WJ. Effects of fibroblast growth factor on type I 5'-deiodinase in FRTL-5 rat thyroid cells. Endocrinology. 1994 Aug;135(2):493-500. [PMID: 7518381]
[34] Hosoi Y, Murakami M, Mizuma H, Ogiwara T, Imamura M, Mori M. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J Clin Endocrinol Metab. 1999 Sep;84(9):3293-300. [PMID: 10487701]
[35] Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J. 2006 Mar;20(3):527-9. [PMID: 16403733]
[36] Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2007 Feb 8;246(1-2):201-9. [PMID: 16563612]
[37] Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006 Jan 15;12(2):662-8. [PMID: 16428513]
[38] Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005 Jan 15;69(2):221-32. [PMID: 15627474]
[39] Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006 Jun;5(6):1434-45. [PMID: 16818501]
[40] Panda S, Kar A. Gugulu (Commiphora mukul) induces triiodothyronine production: possible involvement of lipid peroxidation. Life Sci. 1999;65(12):PL137-41. [PMID: 10503949]
[41] Castillo AI, Sanchez-Martinez R, Moreno JL, Martinez-Iglesias OA, Palacios D, Aranda A. A permissive retinoid X receptor/thyroid hormone receptor heterodimer allows stimulation of prolactin gene transcription by thyroid hormone and 9-cis-retinoic acid. Mol Cell Biol. 2004 Jan;24(2):502-13. [PMID: 14701725]
[42] Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol. 2005 Apr;19(4):863-78. [PMID: 15650024]
[43] Mader S, Chen JY, Chen Z, White J, Chambon P, Gronemeyer H. The patterns of binding of RAR, RXR and TR homo- and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. EMBO J. 1993 Dec 15;12(13):5029-41. [PMID: 8262045]
[44] Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer. 2001;41(1-2):135-44. [PMID: 12094616]
[45] Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001 Aug 15;93(16):1224-33. [PMID: 11504768]
[46] Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J. 2006 Mar;20(3):527-9. [PMID: 16403733]
[47] Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2006 Mar 22. [PMID: 16563612]
[48] Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006 Jan 15;12(2):662-8. [PMID: 16428513]
[49] Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005 Jan 15;69(2):221-32. [PMID: 15627474]
[50] Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006 Jun;5(6):1434-45. [PMID: 16818501]
[51] Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006 Jun 30;281(26):17882-9. [PMID: 16608838]
[52] Leitman DC, Costa CH, Graf H, Baxter JD, Ribeiro RC. Thyroid hormone activation of transcription is potentiated by activators of cAMP-dependent protein kinase. J Biol Chem. 1996 Sep 6;271(36):21950-5. [PMID: 8703000]
[53] Ohmori M, Endo T, Harii N, Onaya T. A novel thyroid transcription factor is essential for thyrotropin-induced up-regulation of Na+/I- symporter gene expression. Mol Endocrinol. 1998 May;12(5):727-36. [PMID: 9605935]
